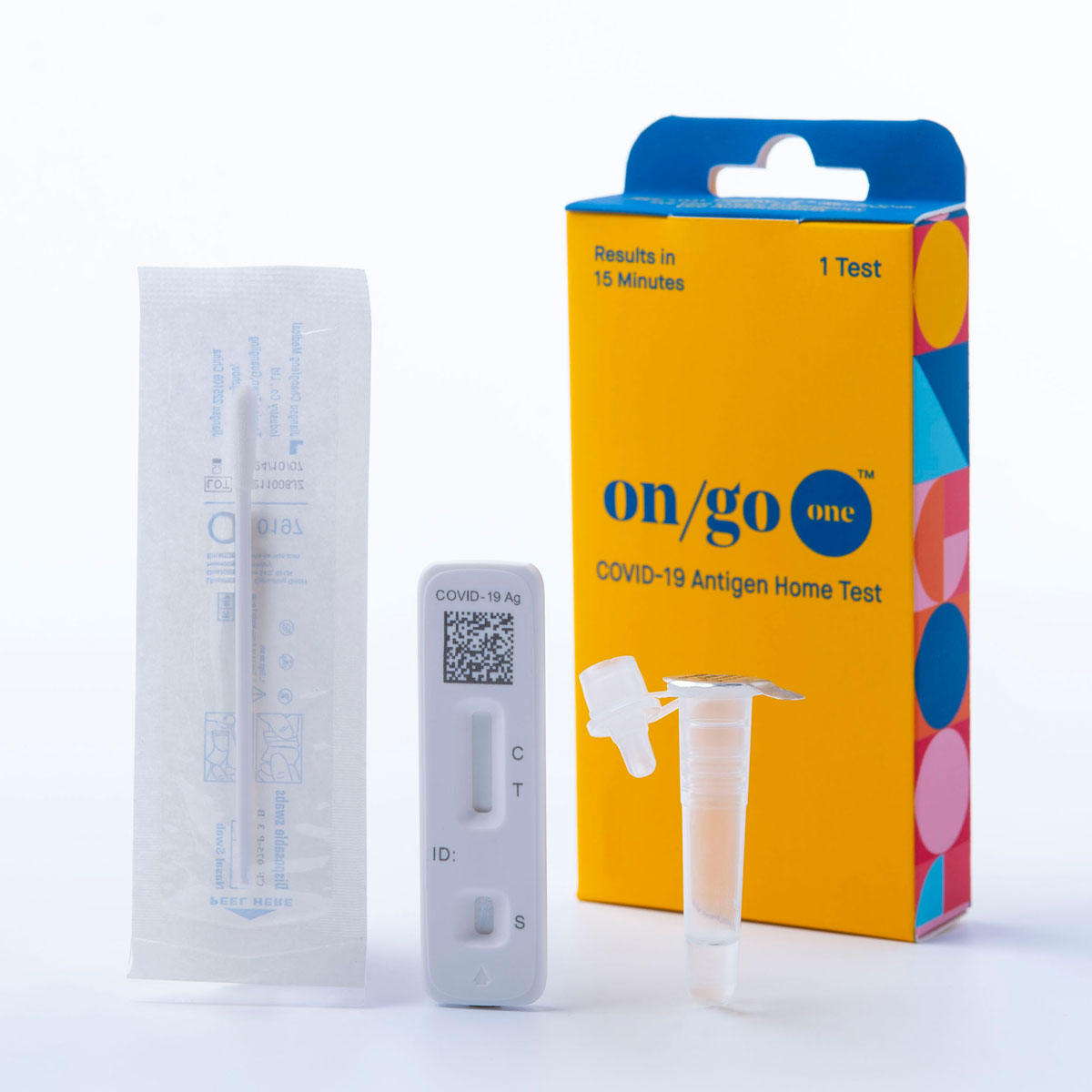
On/Go One Covid-19 Antigen Self-Test
Key Features:
Introducing On/Go One, the single, simple, grab-and-go COVID-19 antigen self-test. Whether you’re going to work, working out, or working it on the dance floor, you gotta have One. On/Go One delivers 98%* accurate test results in just 15 minutes. Plus, it’s covered by most insurance plans, making it as easy on your wallet as it is to use.
- Quick and Easy to Use: The On/Go One COVID-19 Antigen Self-Test by Intrivo uses a shallow nasal swab for maximum comfort, with collection you can perform for yourself or another at home or any place where you can find a flat surface. Indicated for children as young as 2 years old when administered by an adult, and for all people 14 and older to self-perform.
- Quality You Can Trust: In just 15 minutes, On/Go One delivers results with 98%* accuracy. This test detects the antigen protein of all known major COVID-19 variants (including Delta and Omicron). The test works for both symptomatic and asymptomatic individuals.
- Convenient "To-Go" Pack: The small, single On/Go One test is great for easy testing wherever you are going. Put On/Go One in your car, bag, purse, or pocket to always be ready for what’s next!
- Top-Rated Companion App: The On/Go companion app, downloadable by scanning the QR code on the box, guides you seamlessly through each test step, from sample collection to reading your results. The On/Go app is available in the Apple and Google app stores.
- Effortlessly Share Results: With the On/Go app, you can easily track your past results and share them with anyone you choose, making it perfect for events, schools, work, sports and everyday use.
- For more details and NPA/PPA, see On/Go One IFU
Shipping details: Standard shipping included. Expedited shipping available in cart.
Return policy: This item is non-returnable, but if the item arrives damaged or defective, you may request a refund or replacement.
The On/Go and On/Go One tests have not been FDA cleared or approved but have been authorized by FDA under an Emergency Use Authorization (EUA). These products have been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; the tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
The On/Go COVID-19 Antigen Self Test and the On/Go One COVID-19 Antigen Home Tests are lateral flow chromatographic immunoassays intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19.